Description
The earliest known compound of the chemical element argon is argon fluorohydride. In August of 2000, Leonid Khriachtchev, Mika Pettersson, Nino Runeberg, Jan Lundell, and Markku Räsänen reported the synthesis of argon fluorohydride. Argon fluorohydride, which is only stable at very low temperatures, begins to disintegrate as it rises over -246°C. As a result of this constraint, argon fluorohydride has no applications other than basic scientific study.
Chemical Properties
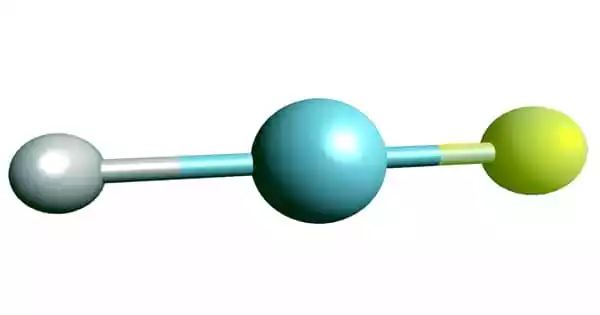
- Chemical formula: HArF
- Chemical structure: H-Ar-F
- Molar mass: 59.954 g/mol
- Appearance: Unknown
- Density: Unknown
- Melting point: −256 °C (decomposes)
- Solubility in water: Unknown
Discovery
This argon compound was discovered by a group of Finnish scientists lead by Markku Räsänen. They published their finding on argon fluorohydride in the journal Nature on August 24, 2000. Although not the first, this finding led to the awareness that argon might form weakly bonded compounds.
Synthesis
By mixing argon and hydrogen fluoride on a cesium iodide surface at 8 K (265 °C) and subjecting the mixture to UV light, this material was formed. The gases mixed as a consequence. When they looked at the infrared spectra of the material, they observed that chemical links had formed, albeit very weak ones, as long as the molecule was kept below 256°C. When heated, it decomposes into argon and hydrogen fluoride.
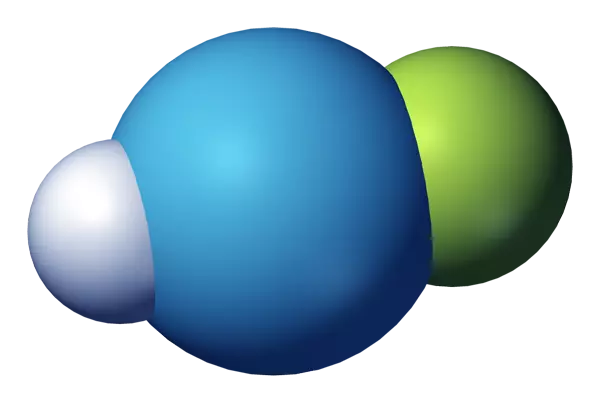
The resultant gas combination’s infrared spectrum indicates the presence of chemical bonds, albeit extremely weak ones; hence, it is argon fluorohydride, not a supermolecule or a mixture of argon and hydrogen fluoride. Its chemical bonds are only stable at temperatures below 27 K (246 °C); when heated, it decomposes into argon and hydrogen fluoride.
Uses
Argon fluorohydride is only stable at very low temperatures, and if it rises above -246 °C (-411 °F), it begins to break down. Outside of fundamental scientific research, argon fluorohydride has no application due to this constraint.
At a pressure of around 400 Pa, it is employed in electric light bulbs and fluorescent tubes, as well as in filling photo tubes, glow tubes, and other similar devices. Argon is also utilized as an inert gas shield for arc welding and cutting, as titanium and another reactive element blanket, and as a protective environment for developing silicon and germanium crystals.