Real laser beams do not interact with one other when they cross, unlike mythical laser swords, unless they meet within a suitable material that allows for nonlinear light-matter interaction. Wave mixing in this situation might result in beams with different hues and orientations.
Wave-mixing processes between various light beams are a cornerstone of the discipline of nonlinear optics, which has grown in prominence since the widespread availability of lasers. Two laser beams may “sense each other’s existence” within a suitable material, such as certain crystals. Energy and momentum can be exchanged during this process, resulting in additional laser beams emanating from the interaction zone in different directions and with varied frequencies, observable as distinct colors in the visible spectrum range. These effects are frequently employed in the design and realization of novel laser light sources.
The investigation of emerging light beams in wave mixing phenomena offers information about the material in which the wave mixing process happens. Researchers can use wave-mixing spectroscopy to learn about the complexities of a specimen’s electrical structure and how light can excite and interact with it. However, these technologies have seldom been applied outside of the visible and infrared spectral ranges.
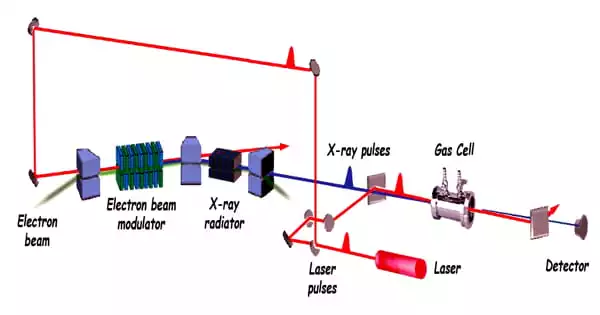
A group of researchers from Berlin’s Max Born Institute (MBI) and Hamburg’s DESY discovered a novel type of wave mixing technique employing soft X-rays. They observed how energy from two infrared photons was transferred to or from the X-ray photon in a so-called third-order nonlinear process by overlapping ultrashort pulses of soft X-rays and infrared light in a single crystal of lithium fluoride (LiF).
They were able to not only witness this process using X-rays for the first time but also map out its effectiveness while altering the hue of the incoming X-rays. It turns out that the mixing signals are only observable when a lithium atom’s inner-shell electron is promoted into a state called an exciton, in which the electron is securely connected to the vacancy it left behind. In addition, a comparison with theory reveals that an otherwise “optically prohibited” inner-shell electron transition contributes to the wave mixing process.
The researchers were able to get a thorough image of where the optically stimulated electron moves in its extremely brief lifetime by analyzing this resonant four-wave mixing process. “We only see the four-wave mixing signal if the excited electron is localized near the hole it has left behind,” says Robin Engel, a Ph.D. student involved in the research. “And because we used a specific color of X-rays, we know that this hole is very similar to the atomic nucleus of the lithium atom.”
The shown technique allows researchers to follow electrons traveling about in molecules or solids after they have been excited by an ultrafast laser pulse. This is because X-rays may selectively activate inner shell electrons at distinct atomic species in a material. These processes—electrons traveling towards various atoms after being energized by light—are essential in photochemical reactions or applications like light-harvesting, such as photovoltaics or direct solar fuel production.
“Many different elements of the periodic table may be selectively stimulated using our wave-mixing spectroscopy method, which can be scaled to considerably higher photon energies using X-ray lasers. We anticipate being able to detect the transitory presence of electrons at many distinct atoms in a more complicated material in this way, providing fresh insight into these critical processes “MBI researcher Daniel Schick adds.